We report the case of a 62-year-old obese patient with untreated dyslipidemia and a smoking history of 30 pack-years, with no other relevant medical history. He presented to the Emergency Department at 7 p.m. with precordial pain at rest, radiating to his upper limbs, rated 8/10 in intensity, lasting 1 hour. Physical examination revealed no relevant findings. The electrocardiogram (ECG) showed ST-segment elevation in the anterior leads and reciprocal ST-segment depression in the inferior leads. The clinical condition was interpreted as anterior ST-segment elevation myocardial infarction (STEMI), Killip and Kimball class A. He received 300 mg of acetylsalicylic acid and 300 mg of clopidogrel. The center did not have an emergency catheterization laboratory, so the patient was transferred to our institution.
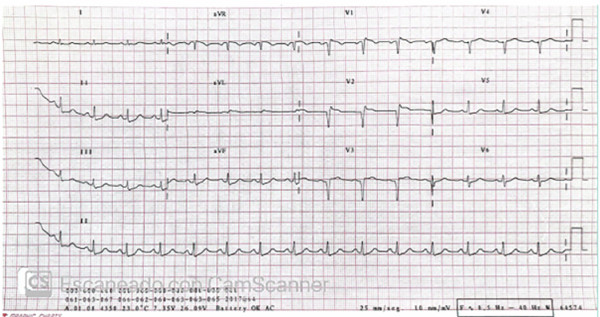
He was admitted at 10 p.m. with persistent precordial pain and an ECG showing the previously described changes (Figure 1). Coronary angiography was performed via left radial artery access, with a door-to-needle time of 10 minutes (Figure 2 A). It revealed occlusion of the left anterior descending (LAD) artery at its origin, and faint opacification of the distal bed through homocoronary and heterocoronary collateral circulation. The lateroventricular branch of the circumflex artery showed an 80% stenosis in the proximal third. The right coronary artery was occluded in the middle third, and its distal bed was opacified through homocoronary collateral circulation.
Fig. 1
Electrocardiogram prior to the first coronary angiography showing ST-segment elevation in the anterior leads and ST- segment depression in the inferior leads.
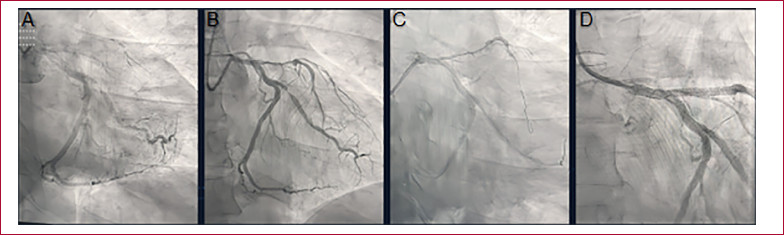
Fig. 2
Coronary angiography (CAG). A. CAG on admission. B. CAG 24 h after treatment with fibrinolytics. C. PCI of the culprit lesion, guidewire in the left anterior descending artery. D. Final angiographic result after stent placement.
Attempts were made to cross the ostial occlusion of the left anterior descending artery using guidewires of different stiffness, but this was unsuccessful. Thromboaspiration was also unsuccessful, so the procedure was concluded at 12:30 a.m. without complications.
The patient was admitted to the Coronary Care Unit after the procedure, with precordial pain rated 6/10. He was alert and had good peripheral perfusion, heart rate 95 bpm, and blood pressure 120/60 mmHg. Heart sounds were normal, with no murmurs; respiratory mechanics was preserved, with no signs of heart failure, and no requirement for supplemental oxygen. Intravenous nitroglycerin infusion was initiated, without improvement in precordial pain. The ECG showed persistent ST-segment elevation.
A bedside echocardiogram showed severe global hypokinesis of the left ventricle, predominantly in the apical segments, without evidence of low cardiac output and with adequate filling pressures.
The team discussed possible strategies for achieving effective reperfusion and, given the patient’s low bleeding risk, a therapy with fibrin-specific fibrinolytics was initiated. Recombinant tissue plasminogen activator (rTPA) was administered at a weight-adjusted dose according to the local protocol: 15 mg over 2 minutes, followed by 50 mg over 30 minutes, and then 35 mg over 60 minutes (total infused dose: 100 mg).
Serial ECGs showed improvement in ST-segment elevation after 30 minutes.
Monitoring from 35 minutes onwards showed frequent ventricular ectopic beats, accelerated idioventricular rhythm (AIVR), and non-sustained monomorphic ventricular tachycardia.
After completion of the rTPA infusion, precordial pain decreased, now rated 2/10, and intravenous nitroglycerin was continued.
At that time, reperfusion criteria were met: decreased ST-segment elevation in V2, (the lead with the greatest elevation), ventricular arrhythmia, and pain relief.
On day 1 of hospitalization, the echocardiogram revealed moderate deterioration of left ventricular function, with a left ventricular ejection fraction (LVEF) estimated at 40% by the Simpson method, akinesia of the mid-septal, mid-anterior, and all apical segments, mild diastolic dysfunction, and a dilated inferior vena cava with minimal collapse.
Twenty-four hours after admission, a new coronary angiography showed an ulcerated lesion in the left main coronary artery and a patent left anterior descending artery, with an extensive severe lesion from its origin to the middle third, showing TIMI II flow (Figure 2 B). The rest of the arteries showed no changes compared to the previous catheterization. During this procedure, PCI was performed on the left anterior descending artery with placement of two drug-eluting stents, on the left main coronary artery with one drug-eluting stent, and on the circumflex artery with two drug-eluting stents.
The procedure was performed via radial access without complications (Figure 2 C and D).
He was readmitted to the Coronary Care Unit, where he developed acute heart failure (Stevenson class B) on day 3 of hospitalization, with a good response to subsequent medical therapy.
After a favorable course without further complications, he was discharged on optimized therapy for coronary artery disease and heart failure. Figure 3 shows the discharge ECG.
DISCUSSION
The treatment of choice for STEMI is invasive, consisting of urgent primary percutaneous coronary intervention (PCI) performed as early as possible. Primary PCI is the recommended reperfusion strategy within the first 120 minutes after the ECG and is superior to fibrinolysis in reducing mortality, non-fatal reinfarction, and stroke. (1)
Fibrinolysis with fibrin-specific drugs is indicated within the first 12 hours of symptom onset, as the start of the pharmacoinvasive strategy, and should be used when PCI is not feasible within the first 120 minutes. (1)
In rare cases, primary PCI may fail. This outcome is associated with higher Killip and Kimball class on admission, multivessel disease, clinical history of acute myocardial infarction (AMI), longer duration of infarction, and TIMI 0-1 flow on admission. (2)
Exceptionally, the culprit vessel cannot be identified. This occurs in the case of ostial lesions, or when the occlusion does not clearly show the stump. (3)
We should consider urgent coronary artery bypass grafting (CABG) for patients with a significant area of myocardium at risk, anatomy unsuitable for PCI, with a patent artery or cardiogenic shock. (1)
The benefits of urgent CABG in patients with PCI failure or acute occlusion not amenable to PCI are uncertain. Therefore, it is not a routine indication, given the high surgical risk of these patients and the low likelihood of improving prognosis. (1)
Primary PCI failure is associated with high morbidity and mortality.
In this setting, the exceptional use of intracoronary fibrinolytics, such as the "marinade technique" has been described, which has few systemic effects, thereby reducing bleeding risk and allowing high drug concentrations at the thrombus site. It has been reported that the use of intracoronary fibrinolytics immediately after PCI could improve coronary perfusion in the first few days after STEMI, improving the area of ischemia and preventing ventricular dysfunction. (4,5)
There is no scientific evidence for "rescue systemic fibrinolysis" (use of systemic fibrinolytics after failed PCI). In our literature search, we found one case in which it was performed in a patient with inferior STEMI. The patient had moderate left ventricular dysfunction. After failed PCI, alteplase was infused achieving reperfusion criteria; then, a PCI of the right coronary artery was successfully performed. (3)
In the case we present, the patient continued to experience pain, with persistent ST-segment elevation, a bedside echocardiogram with a poor prognosis, and an ostial lesion of the left anterior descending artery. As this was a patient with a low risk of bleeding, an off-label drug strategy was chosen to improve the patient's prognosis and eventually reevaluate the coronary arteries with a new angiography.
This clinical case reminds us of the importance of using fibrinolytic treatment according to international guidelines, which is easily accessible and usable in emergency departments that lack emergency primary PCI. It also highlights the current delays in the treatment of STEMI and emphasizes the need for improvements in hospital networks for the management of patients with AMI.
We are used to thinking of rescue PCI in the event of failed fibrinolytic therapy. Although there is no evidence on the reverse procedure-the use of systemic fibrin-specific thrombolytics after failed PCI-, we believe this case has clinical and pathophysiological relevance for exceptional real-world patients with AMI and primary PCI failure.
Conflicts of interest
None declared.
(See conflicts of interest forms on the website).
Ethical considerations
Not applicable