INTRODUCTION
Transthyretin-related cardiac amyloidosis (ATTR- CA) is an infiltrative cardiomyopathy caused by extracellular amyloid protein deposition in cardiac tissue, compromising its structure, ventricular function, and global longitudinal strain (GLS). (1,2,3)
ATTR-CA is an underdiagnosed disease. However, there are clinical, electrocardiographic, and echocardiographic variables that are easily accessible in routine clinical practice and can facilitate early detection. Previously considered a rare disease, diagnosis was made invasively through histological confirmation. However, in the last decade there has been a paradigm shift, driven by the emergence of specific treatments, which has led to research into non-invasive diagnostic methods. (4)
Currently, cardiac scintigraphy with phosphonates is considered a non-invasive biopsy due to its high sensitivity and specificity for diagnosis. It is combined with the assessment of free light chains in blood and urine, allowing for the exclusion of combined or alternative forms of amyloidosis, in particular light chain cardiac amyloidosis (AL-CA).
The amyloid protein has a high affinity for Tc99m-labeled diphosphonate tracers. Devices with cadmium zinc telluride (CZT) detectors evaluate the distribution of hydroxymethylene diphosphonate (HMDP) due to its high resolution. (2,5)
In parallel, it has been postulated that amyloid deposition may affect coronary microcirculation. Myocardial flow reserve (MFR), understood as the ability of the coronary circulation to increase blood flow in response to increased metabolic demand, may be altered by mechanisms related to amyloid infiltration. As amyloid proteins accumulate in the walls of blood vessels and cardiac tissue, their vasoconstrictive and proinflammatory effect may compromise adequate microcirculatory dilation, limiting the heart's ability to adapt to physiological variations. (6,7)
Gamma cameras with CZT detectors have emerged as a noninvasive and accurate alternative for assessing MFR. This method allows the acquisition of dynamic images in order to evaluate the integrated vasodilatory response of the coronary tree through myocardial flow measurement and MFR calculation. (8,9,10)
The primary objective of this study was to evaluate MFR in patients with ATTR-CA and, as a secondary objective, to correlate this parameter with cardiac amyloid distribution and GLS.
METHODS
Study design
This was a single-center, prospective cohort study.
Population
Twenty-two patients with ATTR-CA diagnosed with Grade 3 uptake HMDP on cardiac scintigraphy according to Perugini scale and negative light chains in blood and urine were consecutively included. In all cases, the diagnosis was established exclusively by noninvasive methods, without the need for endomyocardial biopsy.
Patients with known epicardial coronary artery disease were excluded. All participants were not receiving specific treatment at the time of evaluation.
Imaging studies
Conventional echocardiography and myocardial perfusion imaging (MPI) were performed, and Tc99m MIBI was used to assess MFR.
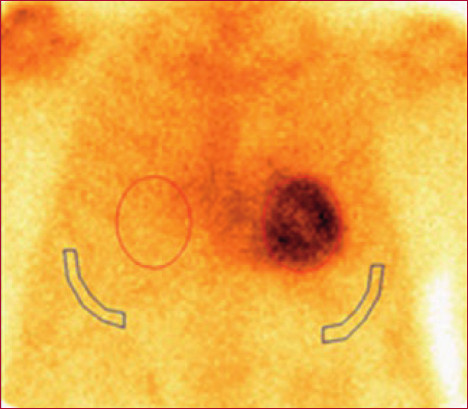
Images with diphosphonates were used to evaluate the degree of overall left ventricular involvement, expressed as percentage, as well as the territorial distribution of amyloid substance. This quantification was performed using polar maps automatically generated by the CZT system software. Uptake values were expressed as relative percentage of total activity in the left ventricle, segmented by coronary territory. (Figure 1)
Fig. 1
The image on the right shows a polar map expressing the distribution of amyloid tissue. The images on the left show tomographic images.
Scintigraphy was performed one hour after intravenous injection of 20 mCi of HMDP-Tc99m. Images were acquired with a dual-head ADAC camera under the following protocol: 1) Planar images (anterior and left anterior oblique): Matrix 128×128 million counts; 2) Gated single-photon emission computed tomography (Gated SPECT): Matrix 64×64 with 30 seconds per frame. The images were processed with Veccsa's VEXWIN software.
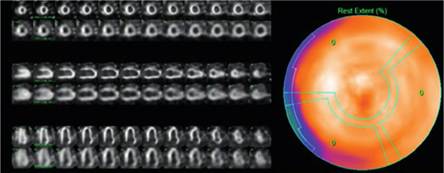
The report was prepared independently by two experienced cardiologists (AM, OM). Discrepancies were resolved by consensus. The degree of cardiac uptake in relation to bone tissue was assessed using two methods: A) Semi-quantitative, following Perugini scale (Figure 2), where cardiac uptake was compared with that of the sternum: grade 0 = no uptake, I = cardiac uptake less than the sternum, II = cardiac uptake similar to the sternum, III = cardiac uptake greater than the sternum, and B) Quantitative: heart-to-lung ratio defined as the number obtained by dividing the number of counts at the level of the heart silhouette by the number of counts in a contralateral area of equal size. (11) (Figure 3)
Fig. 3
Anterior view of cardiac scintigraphy with one region of interest (ROI) located in the cardiac silhouette and another in the contralateral region. The heart-to-lung ratio yields a value of 3.03.
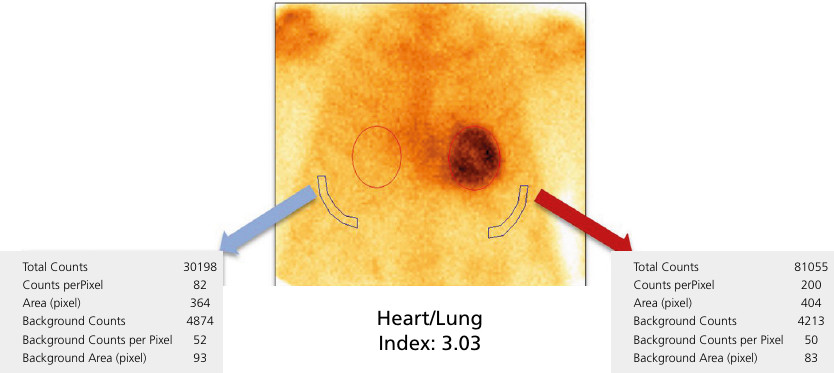
The MFR was determined as the ratio between absolute coronary flow (mL/min/g) during dipyridamole-induced pharmacological vasodilation and flow at rest. A stress flow value greater than1.8 mL/min/g and and MFR ≥ 2 are considered normal. (12) (Figure 4).
Fig. 4
The image on the left shows myocardial flow curves both at rest and during pharmacological stress with dipyridamole. The image on the right shows flow quantification, with peak flow under dipyridamole of 0.77 mL/min/g and at rest of 0.66 mL/ min/g, defining a MFR value of 1.24. MFR: myocardial flow reserve
All patients underwent MPI with MFR assessment using Tc99m-MIBI, with pharmacological stress using dipyridamole and CZT-SPECT acquisition in a one-day protocol, approximately one week after the cardiac scintigraphy with HMDP. Regular medication was not suspended. Patients were instructed to avoid caffeine and proton pump inhibitors for at least 24 hours prior to the study. In addition, a minimum fasting period of two hours prior to the procedure was required.
Baseline hemodynamic values were initially obtained, after which 7 mCi of Tc99m-MIBI were injected at rest and dynamic images were obtained to determine baseline MFR, followed by conventional myocardial perfusion images. At 60 minutes, 0.56 mg/kg of intravenous dipyridamole were administered over 4 minutes, followed by 21 mCi of Tc99m-MIBI, and hemodynamic values and dynamic images were obtained again to determine MFR after stress.
A cardiac color Doppler echocardiogram was also performed to determine ventricular function and GLS. A Philips Affinity C50 ultrasound machine was used. The studies were performed by two operators (PE, MC) with a 5 MHz Matrix transducer to acquire two-dimensional images at a frame rate of 60 to 70 per second. The evaluation of chamber diameters and thicknesses, left atrial area, as well as transvalvular flows with the respective assessments of systolic and diastolic function, was achieved according to the American Society of Echocardiography guidelines.(13) In addition to conventional echocardiographic assessment, GLS was analyzed; from four, three, and two chamber apical views. Processing of 2D strain images was done offline at a workstation.
Statistical Analysis
Categorical variables are presented as percentages and continuous variables as median with their interquartile range (IQR). The normality of continuous variables was assessed using the Shapiro-Wilk test. Since the MFR variable did not have a normal distribution (p <0.05), Spearman's correlation coefficient was used to analyze the correlations between parameters of interest. A p value <0.05 was considered significant. StatsDirect version 3.3.5 was used for the analyses.
Ethical considerations
The study was conducted in accordance with the principles of the Declaration of Helsinki (14) and approved by the institutional teaching and research committee and by an independent ethics committee.
RESULTS
Twenty-two male patients with mean age of 78 ± 7 years, and history of hypertension (82%), dyslipidemia (73%), diabetes (23%), smoking (59%), heart failure (68%), and atrial fibrillation (59%) were included in the study.
Total amyloid involvement in the left ventricle, assessed by SPECT imaging with reconstruction into polar maps, showed a median of 88% (IQR 81%-97%). Territorial quantification of TTR deposition was performed by segmenting the polar maps according to the standardized distribution of the three main coronary territories. A median uptake of 94% (IQR 91%-100%) was observed in the left anterior descending artery territory, 94% (IQR 91%-98%) in the circumflex territory, and 100% in the right coronary territory.
Median left ventricular ejection fraction was 56% (IQR 45%-67.5%) by echocardiography and 52.5%
(IQR 39%-57%) by triggered SPECT, with no statistically significant differences between the two methods.
Median GLS was -8.16 (IQR -9.67 to -6.27).
Myocardial perfusion studies showed no evidence of ischemia or necrosis.
Median MFR was 1.81 (IQR 1.33-2.02) with a peak flow of 1.22 mL/min/g (IQR 0.95-1.74) under stress and 0.77 mL/min/g (IQR 0.64-0.91) at rest
Table 1 shows the individual results of myocardial flow during stress and at rest (mL/min/g), myocardial flow reserve calculated as the ratio between the two, total amyloid deposit extent expressed as percentage, and GLS in the 22 patients with ATTR-CA.
Table 1
Individual results of myocardial flow under stress and at rest (mL/min/g), myocardial flow reserve (stress/rest), total amyloid deposit extent (%), and global longitudinal strain (GLS)
| Patient | Total stress | Total rest | Total reserve | Total extent | GLS |
|---|---|---|---|---|---|
| 1 | 2.29 | 0.88 | 2.62 | 78 | -8.54 |
| 2 | 0.95 | 0.55 | 1.83 | 93 | -6.27 |
| 3 | 1.23 | 0.65 | 1.82 | 88 | -5.74 |
| 4 | 1.65 | 0.83 | 1.89 | 84 | -9.6 |
| 5 | 0.61 | 0.33 | 1.88 | 99 | -6.46 |
| 6 | 0.82 | 0.64 | 1.33 | 96 | -2.87 |
| 7 | 0.79 | 0.50 | 1.71 | 68 | -6.2 |
| 8 | 0.95 | 0.64 | 1.47 | 77 | -6.23 |
| 9 | 2.12 | 1.13 | 1.80 | 80 | -9.67 |
| 10 | 1.99 | 0.74 | 2.61 | 92 | -5.48 |
| 11 | 1.18 | 0.90 | 1.30 | 82 | -7.06 |
| 12 | 1.35 | 0.67 | 2.06 | 99 | -8.33 |
| 13 | 1.12 | 0.86 | 1.31 | 100 | -6.41 |
| 14 | 0.77 | 0.66 | 1.24 | 97 | -8.65 |
| 15 | 1.74 | 0.75 | 2.41 | 100 | -12.3 |
| 16 | 1.74 | 1.08 | 1.61 | 88 | -9.67 |
| 17 | 1.16 | 0.91 | 1.23 | 84 | -12.98 |
| 18 | 1.95 | 0.92 | 2.02 | 91 | -6.41 |
| 19 | 1.22 | 0.94 | 1.30 | 81 | -6.4 |
| 20 | 1.41 | 0.80 | 1.82 | 81 | -11.75 |
| 21 | 1.62 | 0.97 | 1.67 | 85 | -12.96 |
| 22 | 1.21 | 0.46 | 2.81 | 100 | -9.69 |
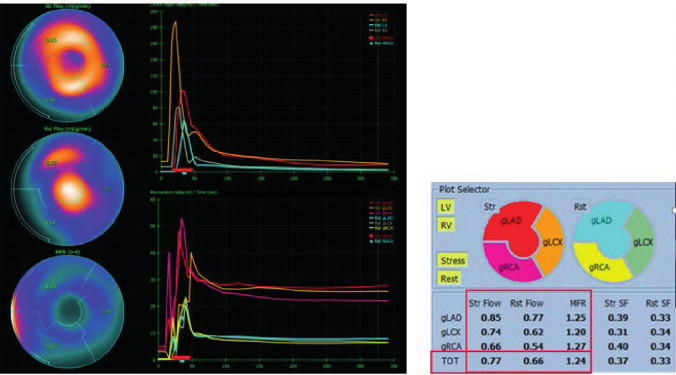
We found no asociation between MFR, amyloid deposit extent, and SLG, with Spearman's correlation coefficients between -0,03 and 0,25, non significant in any case (Figure 5), nor any specific anatomical location of the deposits (Figure 6).
Fig. 5
Polar maps show the percentages of HMDP extension with their corresponding MFR values, demonstrating that there is no correlation between the two parameters.
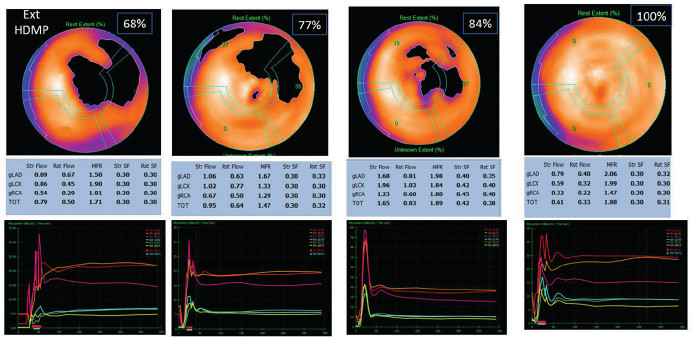
HMDP: hydroxymethylene diphosphonate; MFR: myocardial flow reserve
Fig. 6
Patient’s GLS with apical preservation pattern. The homogeneous distribution of HMDP is observed in the same patient.
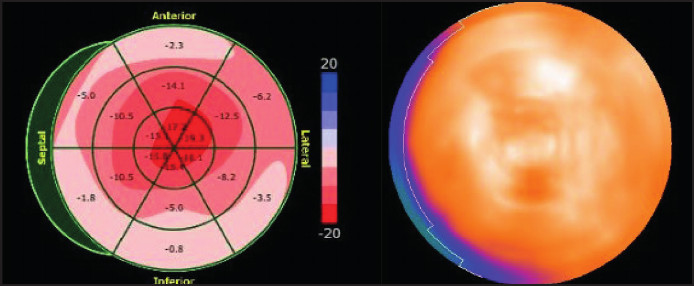
GLS: global longitudinal stress; HMDP: hydroxymethylene diphosphonate
DISCUSSION
In our study, most patients with ATTR-CA had decreased MFR with abnormal peak flow values. We found no association between MFR, cardiac amyloid distribution, and GLS.
The characteristic GLS pattern in cardiac amyloidosis (CA), described as a “Japanese flag,” has traditionally been attributed to the predominant accumulation of amyloid deposits in the basal and mid regions of the ventricle. However, in our study, we observed a homogeneous distribution of deposits, without a specific anatomical preference. (15)
There are no studies in the literature evaluating MFR using CZT cameras. Although there are a few studies addressing MFR, these have been conducted using other methods, such as positron emission to-
mography (PET), echocardiography, and cardiac resonance imaging. Most of these studies include heterogeneous populations, consisting of patients with ATTR-CA and AL-CA, and some consist solely of case reports, which limits the extrapolation of their findings. (16,17,18,19,20)
One of the most relevant studies supporting the role of microvascular dysfunction in cardiac amyloidosis is the prospective study by Dorbala et al., which included a cohort of 21 patients with confirmed diagnosis, 15 with AL-CA and 6 with ATTR-CA, including hereditary and senile forms. All patients were free from significant epicardial coronary artery disease and were compared with a control group with hypertensive left ventricular hypertrophy. Myocardial perfusion at rest and under stress, as well as MFR, were assessed by N-13 ammonium PET and echocardiography. The findings were consistent: patients with CA had significantly reduced myocardial flow at rest and under stress, markedly decreased MFR (1.19 vs. 2.23; p<0.0001), and minimum increased coronary vascular resistance. (20)
A study by Clemmensen et al. specifically evaluated MFR in patients with CA compared with healthy subjects. This prospective cohort included 27 patients with CA, 13 with AL-CA, 9 with hereditary ATTR-CA and 5 with wild-type ATTR-CA. All underwent MFR measurement by transthoracic Doppler echocardiography, evaluating flow in the anterior descending artery during physical exertion in the semi-supine position. The results showed a marked reduction in MFR in patients with CA, with average values of 1.7 vs. 3.9 in the control group (p<0.001). This alteration was consistently observed in the three variants of amyloidosis included in the study, with no significant differences between them. (19)
It is postulated that the underlying pathophysiological mechanisms of microvascular dysfunction in patients with CA could be classified into three main categories: a toxic mechanism, in which the release of
free radicals induced by the amyloid substance generates microcirculatory dysfunction; a vascular mechanism, characterized by the deposition of amyloid in the blood vessel wall; and an extravascular mechanism associated with perivascular and interstitial amyloid deposition. Although these three mechanisms may coexist, the abnormal peak flow values observed in our population suggest microvascular compromise due to extrinsic compression, attributed to interstitial amyloid protein deposition. (15,20,21)
In a recent study that included autopsies performed on patients diagnosed with CA, the histopathological distribution of different types of amyloidosis was analyzed. The findings revealed that in patients with AL-CA the pattern of amyloid fibril deposition was predominantly perivascular. In contrast, in patients with ATTR-CA the predominant pattern was interstitial. These results reinforce what we observed in our population. (22)
The study by Mustafa Bulut et al. proposes that chronic systemic inflammation is responsible for the decrease in MFR in patients with systemic amyloidosis. To verify this hypothesis, MFR was assessed by echocardiography and compared with systemic inflammatory diseases and a control group without disease. The results show that the subgroup of patients with systemic amyloidosis had significantly lower MFR values than other patients with chronic inflammatory diseases without amyloidosis and individuals in the control group. (23)
Published studies have documented a decrease in MFR in patients with cardiac amyloidosis, associating it with the presence of anginal symptoms. It has even been suggested that this alteration could be the first clinical manifestation of the disease. (16,18,24)
The emergence of different specific therapeutic options for CA, many of which are still in the research phase, poses new challenges in clinical practice. Identifying the patients who will benefit most from treatment, choosing the optimal time to start treatment, and selecting the most appropriate drug, or even the possibility of drug combination, given that they act on different pathophysiological mechanisms, are key issues that still require further definition. In this context, measuring MFR could be a useful tool, as it is a functional parameter that has been associated with prognosis and could help stratify patients and guide more personalized therapeutic decisions. (25,26,27)
A prospective multicenter study is currently underway to evaluate MFR using CZT cameras in patients with ATTR-CA diagnosis free from previous treatment, with baseline measurement and reassessment two years after initiation of tafamidis therapy. Although the results have not yet been published, this study is expected to provide relevant information to further investigate the pathophysiological mechanisms involved and the functional response to specific treatment, important aspects that remain poorly explored in this population. (28)
Our findings reinforce the presence of microvascular dysfunction in patients with ATTR-CA. The reduction in MFR observed in our population, associated with a homogeneous distribution of amyloid deposits and marked deterioration of GLS, suggests that functional compromise of the coronary tree may represent an early manifestation independent of the degree of structural infiltration. Although no correlation was found between MFR and amyloid burden, this observation raises new pathophysiological and methodological questions that deserve further exploration. The use of noninvasive tools, such as CZT cameras, in combination with more accurate tissue quantification techniques, could open new opportunities for risk stratification and therapeutic monitoring in this population.
CONCLUSION
Myocardial flow reserve is compromised in patients with ATTR-CA diagnosis and abnormal peak flow values, suggesting microvascular involvement due to interstitial amyloid protein deposition. However, it was not possible to associate MFR compromise with amyloid tissue burden in this population.
We found no correlation between MFR, cardiac amyloid distribution, and GLS.
Myocardial flow reserve assessment could become a tool for understanding disease progression and stratifying risk in patients with ATTR-CA.
Acknowledgments
Our sincere and heartfelt thanks go to the entire technical team, whose invaluable contribution and constant dedication have been fundamental for the completion of this work. Without their efforts, this project would not have been possible;
Limitations
The study population was small and in advanced stages of the disease.
Conflicts of interest
None declared.
(See authors' conflict of interests forms on the web).