INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide (1) and ranks first among the causes of disability in people > 50 years of age according to the Global Burden of Disease Study (2019). (2) Hypercholesterolemia remains one of the major determinants of attributable risk for CVD and cerebrovascular disease. (3)
In Argentina, the INTERASPIRE registry found that, during follow-up, 55% of the population who had a first coronary event did not meet the cholesterol bound to low-density lipoproteins (LDL-C) targets established by clinical practice guidelines. (4) It is known that the key event that triggers atherogenesis is the retention of LDL-C and other cholesterol-rich lipoproteins. These lipoproteins carry the cholesterol within the artery wall, thereby playing a pivotal role in the formation of atherosclerotic plaque and the subsequent development of CVD. Increased LDL-C concentration is causally related to atherosclerotic cardiovascular disease, and reducing the number of LDL-C particles and other lipoproteins containing apolipoprotein B (apoB) as much as possible decreases the occurrence of major adverse cardiovascular events (MACE). (5,6) The current problem is that despite optimal control of LDL- C levels through lifestyle changes and lipid-lowering treatment, patients who survive a first CVD event are at high risk of recurrence. (7) This can be attributed to the failure to consider residual cardiovascular risk due to elevated levels of other atherogenic particles: non- HDL cholesterol (non-HDL-C), remnant cholesterol (RC), and lipoprotein(a) [Lp(a)]. (8,9)
Non-HDL-C comprises all plasma lipoproteins except HDL-C: LDL-C, triglyceride-rich lipoproteins (TRL), which include intermediate-density lipoproteins (IDL), very low-density lipoproteins (VLDL), chylomicrons, remnant TRL, and Lp(a). This parameter indicates the total amount of lipoproteins containing apolipoprotein B. (10) Non-HDL-C has been shown to be more effective for estimating the risk of atherosclerotic cardiovascular disease when compared to LDL-C. This may be due to the fact that it encompasses all proatherogenic cholesterol particles. (10) It is also a superior predictor of risk in patients with metabolic disorders, including hypertriglyceridemia, diabetes mellitus (DM), and obesity.
In secondary prevention, the desirable value of non-HDL-C is < 85 mg/dL for patients with very high CV risk associated with DM, acute coronary syndrome (ACS), familial hypercholesterolemia (FH), recurrent
events, and panvascular disease. For patients without these conditions, the desirable value is < 100 mg/dL. (12,13) RC is the cholesterol content of TRLs, and can be estimated as total cholesterol minus LDL-C minus HDL-C. Like non-HDL-C, RC is a causal factor in CVD and has been shown to have the potential to predict CVD independently of LDL-C levels. (12) The evidence from randomized clinical trials supports the hypothesis that elevated levels of these particles are associated with an increased risk of myocardial infarction (MI) and all-cause mortality. (14,15)
It is also known that the residual risk in these patients is multifactorial and is not only related to lipid factors but also to the persistence of other risk factors such as increased body mass index (BMI), hypertension (HTN) and diabetes mellitus (DM). (8,9) The primary objective of this study is to determine the role of non-HDL-C and RC as predictors of recurrent atherosclerotic cardiovascular events in patients with ST-segment elevation myocardial infarction (STEMI).
METHODS
Study design and population
We conducted a retrospective cohort study at a high-complexity center in the city of Buenos Aires. Patients > 18 years who were hospitalized due to STEMI with evidence of type 1 MI as demonstrated by invasive coronary angiography were included in the study. The diagnosis of STEMI was based on the fourth universal definition of MI, (16) with patients presenting with electrocardiographic changes consistent with ST-segment elevation at the J point in at least two contiguous leads, > 0.25 mV in men < 40 years, > 0.20 mV in men > 40 years or older, or > 0.15 mV in women in leads V2-V3 and/or > or equal to 0.10 mV in all leads.
Total cholesterol (TC), HDL-C, and triglyceride (TG) levels were measured in venous blood samples obtained within the first 12 hours following hospital admission. Samples were obtained in a fasting state (minimum 8 hours) when possible, but, this condition was not recorded. LDL-C was estimated using the Friedewald formula: LDL-C = TC - HDL-C - (TG/5), expressed in mg/dL. Non-HDL-C was calculated as the difference between TC and HDL-C, and RC was calculated using the formula TC - HDL-C - LDL-C. Given the elevated cardiovascular risk exhibited by these patients, the therapeutic targets were defined as LDL-C of <55 mg/dL or a reduction of at least 50% from baseline levels.
The primary outcome was a composite of unstable angina and major adverse cardiovascular events (MACE), defined as cardiovascular death, nonfatal stroke, or nonfatal MI during the first 12 months of follow-up after the index event. Follow-up was mainly performed through electronic medical records. Those patients who were not followed up at our institution were contacted by telephone calls.
Statistical analysis
All the statistical calculations were performed using RStudio version 1.4.1106 (The R Foundation for Statistical Computing, Viena, Austria). Continuous variables are expressed as mean and standard deviation, or median and interquartile range (IQR), according to their distribution. Qualitative variables are presented as absolute frequencies and percentages. The chi square test or Fisher's exact test were used to compare the categorical variables and continuous variables were analyzed using the Student's t test or the Mann-Withney test, depending on the distribution of the sample. Cox regression analysis was used to identify independent predictors of cardiovascular events during follow-up. All models were adjusted for age, sex, HTN, DM, smoking habit, RC, LDL-C, and non-HDL-C. Survival curves were estimated using the Kaplan-Meier method. A p-value < 0.05 was considered statistically significant.
RESULTS
Patients' characteristics
A total of 403 patients with STEMI were included; median age was 64 years (IQR 55-73) and 78.8% were male. The main cardiovascular risk factors among the patients included were DM (16%), HTN (43%), dyslipidemia (29%) and current smoking (12%). Median values of LDL-C , HDL-C , TG, and non-HDL-C were 64 mg/dL, 36 mg/dL, 114 mg/dL, and 87 mg/dL,
respectively. During the average follow-up period of 12 ± 3 months, adherence to statin treatment was 97.8%
Events at follow-up according to the change in non-HDL- cholesterol
The cardiovascular outcome occurred in 95 patients (23.5%) during follow-up.
Patients who developed MACE had higher levels of TC, TG,non-HDL-C and RC. There were no differences in LDL-C levels. The characteristics of the population and their relationship with the occurrence of events can be seen in Table 1. In a stratified analysis, patients who experienced events during follow-up had higher non-HDL-C and RC levels compared to those without events, despite having the LDL-C target value (Figure 1).
Table 1
Baseline characteristics of the population and relationship with major adverse cardiovascular events (MACE)
| MACE | ||||
|---|---|---|---|---|
| Variables | Total | No | Yes | p |
| Patients | 403 (100) | 308 (76.5) | 95 (23.5) | |
| Male | 316 (78.8) | 250 (81.7) | 66 (69.5) | 0.016 |
| Age, years | 64 (55-73) | 63 (54.71) | 67.50 (57, 77) | 0.009 |
| DM | 67 (16.6) | 54 (17.5) | 13 (13.7) | 0.470 |
| TBQ | 50 (12.4) | 46 (14.9) | 4 (4.2) | 0.009 |
| DLP | 118 (29.3) | 84 (27.3) | 34 (35.8) | 0.143 |
| HTN | 175 (43.4) | 127 (41.2) | 48 (50.5) | 0.139 |
| Adherence to statins | 394 (97.8) | 303 (98.4) | 91 (95.8) | 0.274 |
| LDL-C | 64 (50-85) | 64 (50-80) | 65 (52-94) | 0.327 |
| HDL-C | 36 (30-44) | 35 (30-42) | 39 (30-48) | 0.151 |
| TG | 114 (84-69) | 109.50 (79-160) | 140 (100-196) | <0.001 |
| TC | 124 (103-49) | 121 (102-140) | 138 (118-177) | <0.001 |
| Non-HDL-C | 87 (70-110) | 84 (69-105) | 102 (75-135) | <0.001 |
| RC | 22 (13-33) | 20 (14-31) | 29 (17-48) | <0.001 |
| Follow-up in months | 4 (1-10) | 6 (1-11) | 1 (1-4) | <0.001 |
Qualitative variables are presented as frequency and percentage. Quantitative variables are presented as median and interquartile range or mean DLP: dyslipidemia; DM: diabetes mellitus; HDL-C: high-density lipoprotein cholesterol; HTN: hypertension; LDL-C: low-density lipoproteins cholesterol; Non-HDL-C: cholesterol not associated with high-density lipoproteins; RC: remnant cholesterol; TBQ: tobacco use; TC: total cholesterol;TG: triglycerides
Fig. 1
A: shows the relationship between RC and non-HDL-C according to LDL-C levels. Both groups exhibit a positive linear association between the two variables. However, the slope corresponding to the group with LDL-C < 55 mg/dL (blue line) is above the red line, indicating that, for the same RC value, these patients had higher levels of non-HDL-C. B: shows the comparison of non-HDL-C levels according to the occurrence of events (MACE), stratified by LDL-C levels. In both strata, patients who experienced events (red) had significantly higher levels of non-HDL-C compared to those without events (blue), with statistically significant differences.
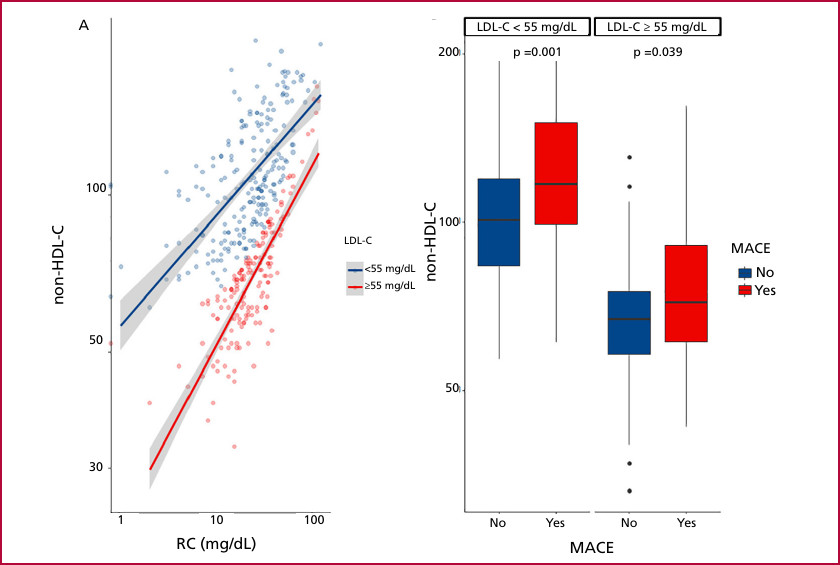
LDL-C: low-density lipoprotein cholesterol; MACE: major adverse cardiovascular events; non-HDL-C: non-high-density lipoprotein cholesterol; RC: remnant cholesterol.
Multivariate analysis: independent predictors of major cardiovascular events
Two prediction models were adjusted for Cox multiple regression, one with the LDL-C variable and the other with the non-HDL-C variable. The hazard ratio (HR) for LDL-C was 1.15 (95% CI: 0.94-1.39, p=0.166), while the HR for non-HDL-C was 1.45 (95% CI: 1.21-1.73, p < 0.001) (Figure 2). Patients with non-HDL-C levels within the target range had higher event-free survival at follow-up (p=0.001) (Figure 3).
Fig. 2
Cox regression model for major adverse cardiovascular events (MACE)
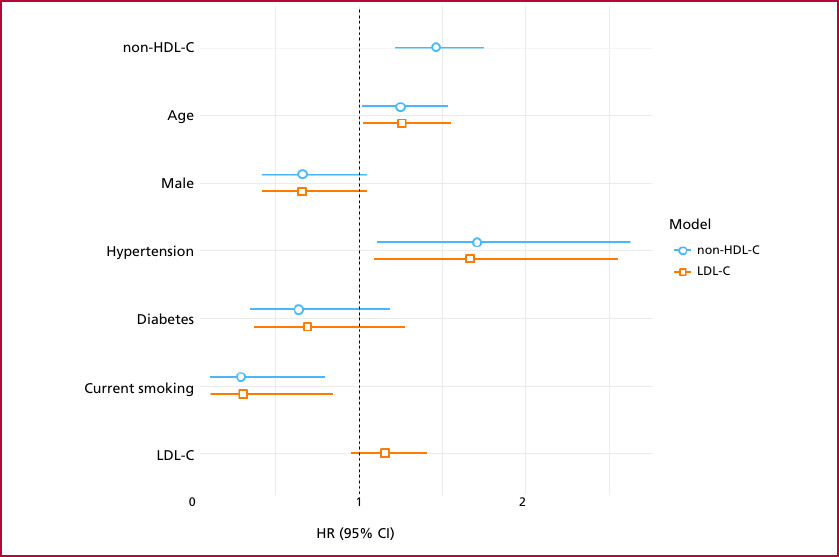
95% CI: 95% confidence interval; HR: hazard ratio; LDL-C: low-density lipoprotein cholesterol; non-HDL-C: non-high-density lipoprotein cholesterol
Fig. 3
Major adverse cardiovascular events (MACE) according to non-HDL-C target
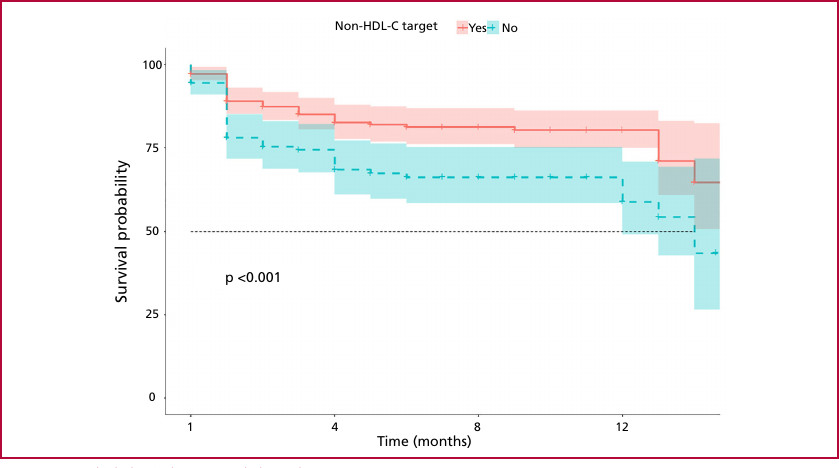
non-HDL-C: non-high-density lipoprotein cholesterol
DISCUSSION
During the first year of follow-up after the index event, 23.5% of the patients included experienced MACE. There were no significant differences in the occurrence of events according to LDL-C levels. Conversely, patients with MACE did not achieve non- HDL-C and RC target levels, with median values of 102 mg/dL and 29 mg/dL, respectively. Furthermore, non-HDL-Cl was identified as an independent predictor of MACE during follow-up, in contrast to LDL-C, which did not demonstrate such an association.
In the SWEDEHEART registry, (17) which included 56 262 patients with MI as their first cardiovascular event, 17% of patients experienced MACE during follow-up. The efficacy of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, alirocumab and evolocumab, compared with placebo, in reducing LDL-C and decreasing MACE was evaluated in two clinical trials (ODYSSEY and FOURIER). Despite achieving critical levels of LDL-C reduction (with medians of 38 mg/dL and 30 mg/dL, respectively), the incidence of MACE during follow-up in patients receiving monoclonal antibodies was 9.5% and 12.6%, respectively. (18,19) In our cohort, median LDL-C in patients with a new event during follow-up was 64 mg/dL, very close to the target value suggested by international guidelines.
The meta-analysis by Boekholdt et al. (20) showed a strong association between LDL-C and non-HDL-C values as predictors of MACE at follow-up, but the association was stronger for non-HDL-C. Even in those patients who achieved LDL-C targets but did not do so for non-HDL-C, the HR was 1.32 (95% CI 1.17-1.50; p < 0.001). In the aforementioned Swedish registry, (17) baseline non-HDL-C levels were measured at the time of admission and at 1-year follow-up.The cumulative incidence rates by quartile of non-HDL-C reduction showed a clear separation of the survival curves. This finding demonstrates that a greater reduction in non-HDL-C (≥ 85 mg/dL) was associated with a lower rate of adverse events at follow-up. Patients in the quartile with the greatest reduction exhibited a 37 % decrease in the risk of MACE (HR 0.63; 95 % CI 0.57-0.68), as well as a 21 % reduction in the risk of all-cause mortality and a 49 % reduction in the risk of nonfatal MI (p < 0.001). In addition, the risk of MACE at one year of follow-up was significantly lower in patients who achieved the non-HDL-C target earlier and maintained it during the first year of follow-up (HR 0.80; 95% CI 0.74-0.86). These results underscore the importance of initiating treatment and achieving non- HDL-C targets early after the index event to optimize long-term clinical outcomes. These findings are similar to those observed in our study, in which non-HDL- C performed as an independent predictor of events with a HR of 1.45 (95% CI 1.21-1.73, p <0.001).
Two randomized clinical trials analyzed discrepancies between apoB, non-HDL-C, and LDL-C levels to assess residual risk of MI. Elevated levels of apoB and non-HDL-C, but not of LDL-C levels, were associated with an increased risk of MI and all-cause mortality. In contrast, elevated LDL-C with discordant low apoB or non-HDL-C levels, did not show such an association. (7,21,22,23) Although our study did not include apoB measurement, these findings underscore the importance of considering other lipid parameters in addition to LDL-C for better assessment of the residual risk in our patients.
In our study, LDL-C did not result a significant predictor of events; yet, its clinical role remains fully valid. LDL-C remains a primary therapeutic target in the field of cardiovascular prevention, with substantial evidence from multiple studies and registries supporting its intensive reduction. According to the leading international guidelines, such as those of the American College of Cardiology (ACC) and the European Society of Cardiology (ESC/EAS), it is recommended that patients at very high cardiovascular risk achieve LDL-C levels of less than 55 mg/dL. (6,24) Therefore, our findings should not be interpreted as a disregard of the clinical importance of LDL-C. Rather, they should be seen as a call to broaden the focus to include other relevant lipid parameters in the assessment of residual risk.
Study limitations
Our study has several limitations that deserve consideration. First, given the retrospective nature of the study, complete information on lipid-lowering treatment, medication adjustment during follow-up, and patient adherence was not available. These data were collected from the electronic medical record and by telephone contact in specific cases. Second, patient recruitment and follow-up were limited to a single center, so our results represent the reality of the partici-
pating center. Third, LDL-C was estimated using the Friedewald formula, which is a methodological limitation that can lead to loss of diagnostic accuracy in the presence of moderate or high hypertriglyceridemia. This situation, which is common in patients with high cardiovascular risk, could have led to an underestimation of the actual LDL-C value, particularly in those cases with TG levels ≥ 200 mg/dL. Finally, we did not include the baseline lipid profile, which prevented us from calculating the absolute and percent reductions of the different lipid fractions during follow-up.
CONCLUSION
Our study highlights that, even with LDL-C levels close to the targets recommended by the guidelines, a significant percentage of patients still face a high risk of major cardiovascular events. Non-HDL-C proved to be a key marker for identifying this residual risk, underscoring the need to consider it as a complementary tool for optimizing prevention and management strategies in these patients.
Conflicts of interest
None declared.
(See authors' conflict of interests forms on the web).
